Abstract
Background: Despite the high complete remission (CR) rates achieved with IC, relapse rates remain high underscoring the need for novel combination regimens capable of inducing deeper remissions via eradicating measurable residual disease (MRD). Recently, several studies have shown promising clinical activity of VEN when combined with different IC regimens in pts with ND AML with composite CR rates (CRc) above 90% and MRD-ve rates exceeding 80% (Wang 2022; Kadia 2021). FLAG-IDA + VEN has also shown significant clinical activity in pts with ND AML with a CRc rate of 89% and MRD-ve remission rate of 93% (DiNardo 2022).
Methods: We conducted a single-center retrospective study to assess the clinical activity of FLAG-IDA + VEN in pts with AML. During induction, VEN was initially given for 14 days which was later on reduced to 7 days due to encouraging results by Kadia et al. Standard cytogenetic (CN) analysis was performed and molecular data was obtained using a 40-gene NGS platform. Responses were assessed using the modified International Working Group response criteria and included CR, CR with incomplete count recovery (CRi), morphologic leukemia-free state (MLFS), and partial remission (PR). Time-to-event analyses were estimated using the Kaplan-Meier method and compared using the log rank test.
Results: From March 2020 to May 2022, 24 pts with AML (17 ND and 7 R/R) were treated using FLAG-IDA + VEN (Table 1). Median age was 48 years (range, 21-68) with male predominance (67%). Among the 17 pts with ND AML (8 de novo, 5 AML-MRC, 2 treated-secondary, 2 therapy-related), 3 had extramedullary disease (EMD). The most common ELN CN risk groups were complex (N=5), KMT2A-rearranged (N=4), other adverse (N=3), and diploid (N=3). Eight pts (47%) harbored a RAS mutation. Median time to initiation of therapy was 5 days (range, 2-14). Twelve pts (71%) [KMT2A-rearranged, N=4; complex CN, N=3; diploid, N=3; other adverse, N=1; other intermediate, N=1] achieved CR/CRi including 1 pt with EMD. Median time to ANC (≥ 500) and platelet (≥ 50,000) recovery were 29 days (range, 19- 42) and 25 days (range, 17- 42), respectively. Median number of consolidation cycles given were 1.5 (range, 1- 5). MRD status was available in 6 out of 12 pts and all 6 pts achieved MRD negativity using flow cytometry (<10-3). Median follow-up among ND AML pts was 4.3 months (mths) (range, 0.7- 13.2). Among the 12 responders: (1) 3 pts received allo-SCT [KMT2A-rearranged, N=2; (-7), N=1] after 7, 4, and 3 mths, respectively, and remain alive in CR 10+, 7.5+, and 3+ mths, respectively, after initiation of therapy, (2) 6 pts with ELN favorable (N=3), intermediate (N=2), and adverse (N=1) risk AML remain in CR/CRi for 6+, 4+, 1+, 3+, 2+, and 2+ mths, respectively, (3) 3 pts with ELN adverse risk AML (including 1 pt with de novo MLLr AML and EMD) achieved CR and relapsed after 0.7, 1.7, and 7.6 mths, respectively. One pt died due to progressive disease (PD) 4.7 mths after relapse and the remaining 2 pts are receiving salvage therapy and alive 4.3+ and 5.2+ mths after relapse, respectively. All nonresponders (N=5) had ELN adverse risk AML including 2 pts with EMD and 2 pts with treated secondary AML. All 5 nonresponders died due to PD including 3 pts who had no response to HMA+VEN based salvage therapy.
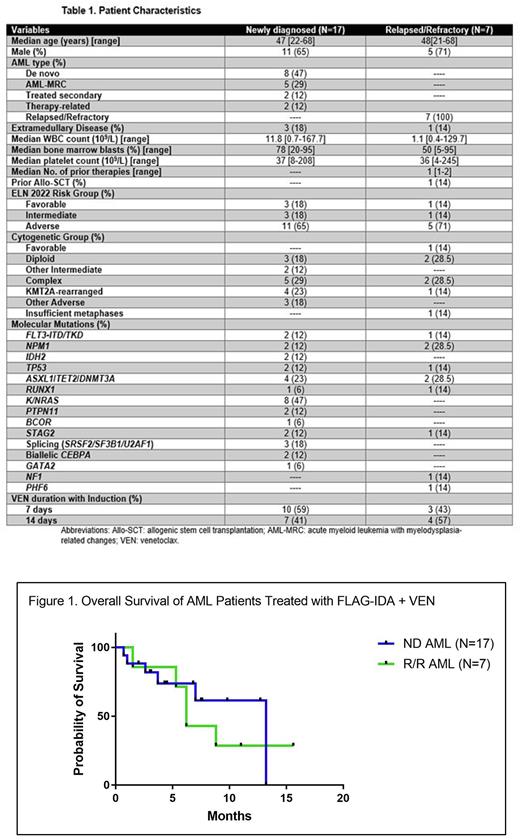
Among the 7 pts with R/R AML, 6 were in salvage 1 and 1 pt received prior allo-SCT. Responses were observed in all pts: 4 CR, 1 CRi, 1 MLFS, and 1 PR. Median time to count recovery in R/R AML pts was 33 days (range, 24-78). Median follow-up in R/R AML pts was 6.2 mths (range, 1.5- 15.6). Of the 5 pts who achieved CR/CRi, 4 were bridged to allo-SCT and 1 pt died in CR after 3 cycles of consolidation due to sepsis. Of the 4 SCT recipients: (1) 2 pts relapsed 2 mths after allo-SCT and died 4 and 6.5 mths, respectively, after allo-SCT; (2) 2 pts (KMT2A-rearranged and NPM1mut) remain alive in CR for 8+ and 14+ mths, respectively. Two pts with complex CN achieved MLFS and PR after 1 cycle of therapy and died due to PD and fungal pneumonia after 5.3 and 1.5 mths of starting therapy, respectively. Median overall survival (OS) for pts with ND AML and R/R AML were 13.2 mths (95% CI: Not reached) and 6.2 mths (95% CI: 5-7), respectively (p=0.62; Figure 1). Among pts with ND AML, median OS was not reached among responders and was 2.6 mths in non-responders (p=0.002).
Conclusion: FLAG-IDA + VEN is a feasible and active regimen in AML. Longer follow-up and larger multicenter studies are needed to confirm the efficacy of this regimen.
Disclosures
Abaza:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; ALX Oncology: Research Funding. Altman:Fujifilm: Research Funding; Kartos Therapeutics: Research Funding; Kura Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Glycomimetics: Other: Data Monitoring Committee; Loxo: Research Funding; ImmunoGen: Research Funding; Biosight: Membership on an entity's Board of Directors or advisory committees, Other: reumbursement for travel, Research Funding; Celgene: Research Funding; Boehringer Ingelheim: Research Funding; Astellas: Honoraria, Research Funding; Aptos: Research Funding; Aprea: Research Funding; Amgen: Research Funding; Abbvie: Honoraria, Research Funding; ALX Oncology Inc: Research Funding; Syros: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Venetoclax is currently approved in combination with azacitadine for patients with newly diagnosed AML unfit for intensive chemotherapy. To date, venetoclax has not yet been approved in combination with intensive chemotherapy for the treatment of patients with AML fit for intensive chemotherapy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal